Introduction
Immune checkpoint inhibitors (ICIs) are a highly effective class of cancer immunotherapy, but their use has been limited by immune-related adverse events (irAEs) affecting various organ systems. Recent real-world studies have shown higher incidence rates of cardiovascular (CV) events in patients with cancer who are treated with ICIs compared to those treated with conventional chemotherapy (Laenens et al, J Clin Oncol 2022). Studies suggest that the underlying mechanism involves a pro-inflammatory response causing unintended vascular compromise, but there have been limited data on ICI-related cardiotoxicity across pooled datasets. Given that ICIs have been applied to lung cancer treatment in recent years, we use it as an example indication to study clinical trial (CT) patients with advanced non-small cell lung cancer (aNSCLC) randomized to either a first-line (1L) ICI-containing regimen or to 1L chemotherapy alone (chemo) to evaluate for potential differential rates of CV events.
Methods
We pooled patient-level, phase 3 CT data on aNSCLC patients treated with ICI or chemo from the Medidata Enterprise Data Store. Treatment assignment was the main variable of interest. The primary outcome was a composite CV event (acute coronary syndrome, heart failure, stroke, transient ischemic attack), occurring between the start of study treatment (index date) and 30 days following the last dose of study treatment. Incidence rates and the relative hazard of a CV event by treatment assignment were estimated. Additionally, the risk of CV event with non-CV death treated as a competing risk was estimated using the Fine-Gray model. In a post-hoc subgroup analysis we explored the impact of treatment assignment on overall survival (OS) among patients with and without baseline vascular disease. OS was defined as the time from the index date until the date of death (all-cause). Patients alive at the end of CT follow-up were censored.
Results
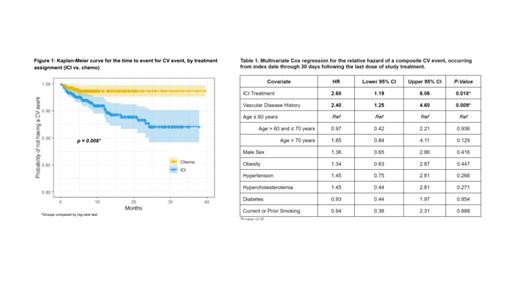
A total of 1862 patients were included in this analysis, with 1128 (60.6%) randomized to ICI and 734 (39.4%) to chemo. Baseline demographics and comorbidities were balanced across treatment groups. Forty-six (2.5%) patients experienced at least 1 CV event. The incidence rate of CV events was 2.48 times (95% CI: 1.10, 6.57) higher among patients treated with ICI compared to chemo. The unadjusted hazard of composite CV event, estimated using Kaplan-Meier, differed significantly by treatment assignment (p= 0.008) ( Figure 1). After adjusting for baseline demographics and comorbidities, the hazard of a composite CV event remained higher for patients treated with ICI compared to chemo (aHR 2.68, 95% CI: 1.19-6.06) ( Table 1). Baseline vascular disease history (N=359; 19.3%) was also associated with an increased hazard of a CV event (aHR: 2.40; 95% CI: 1.25-4.60). Similar results were found in the competing risk analysis. In the subgroup analysis, patients without baseline vascular disease history had a significant OS benefit from an ICI compared to chemo (aHR 0.84, 95% CI: 0.75-0.95), whereas patients with baseline vascular disease did not (aHR 1.00, 95% CI: 0.78-1.27).
Conclusion
Though ICIs enhance the body's immune responses against cancer, it is important to study the associated effect of irAEs on off-target tissues. These analyses of historical CT data suggest a positive association between 1L treatment of aNSCLC with ICI and subsequent composite CV events compared with chemo alone. Given the high prevalence of morbidity among patients with lung cancer, the use of concomitant cardioprotective therapies may be an important consideration when treating cancer populations with ICIs.
Pooling CT data increases the power to detect clinically important differences in rare toxicities and may inform future research and patient-physician decision-making regarding the suitability of ICI use in aNSCLC treatment. Furthermore, this research highlights the potential clinical utility of drug repurposing: expanding the use of drugs approved to treat CV events and exploring their use in patients with vascular disease who are also being treated with ICIs. As cell-mediated immune responses play a key role in the potential CV effects of ICI treatments, future studies are needed to define the underlying mechanisms of ICI-related dysregulation of the immune system to better monitor patients and improve clinical outcomes in the context of immunotherapy for aNSCLC and other cancer types.
Disclosures
Ports:Medidata, a Dassault Systemes company: Current Employment. Jain:Medidata, a Dassault Systemes company: Current Employment. Diamond:Medidata, a Dassault Systemes company: Current Employment. Aptekar:Medidata, a Dassault Systemes company: Current Employment. Fajgenbaum:Medidata, a Dassault Systemes company: Consultancy; EUSA Pharma/Recordati Rare Disease: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal